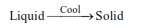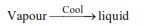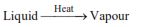Introduction
Introduction
The concept of heat and temperature are studied together in science. The terms are related to each other, due to their wide usage in our day to day life. However, heat and temperature are different concepts. Heat, q, is thermal energy transferred from a hotter system to a cooler system that are in contact. Temperature is defined as the degree of hotness or coldness of a body.
Heat vs. Temperature: All molecules contain some amount of kinetic energy, that is to say, they have some intrinsic motion. The Heat of an object is the total energy of all the molecular motion inside that object. Temperature, on the other hand, is a measure of the average heat or thermal energy of the molecules in a substance.
- Heat is the transfer of kinetic energy from one medium or object to another, or from an energy source to a medium or object.
- Temperature is defined as the degree of hotness or coldness of a body. It is a scalar quantity.
- Its S.I. unit is kelvin (K).
- Heat is a form of energy which causes sensation of hotness or coldness. The flow of heat is always from higher temperature to lower temperature. No heat flows from one body to other, when both the bodies are at the same temperature. The two bodies are said to be in thermal equilibrium.
- The SI unit of heat is joule.
- Its CGS unit is calorie, 1 cal = 4.2 joule
 Temperature
Temperature
- A branch of science which deals with the measurement of temperature of a substance is called thermometry.
- Thermometer is a device used to measure the temperature.
- Thermometer used for measuring very high temperatures are called pyrometer. Relationship Between Different Scales of Temperature [latex]\frac{C - 0}{100}[/latex]= [latex]\frac{F - 32}{212 - 32}[/latex]=[latex]\frac{K - 273.16}{373.16 - 273.16}[/latex] = [latex]\frac{R - 0}{80 - 0}[/latex] = [latex]\frac{Ra - 460}{672 - 460}[/latex] [latex]T^°(K)[/latex]= [latex](t^°C + 273.16)[/latex] Normal temperature of human body is 310.15 k [latex](37^°C = 98.6^°F)[/latex] STP or NTP implies 273.15 K[latex](0^° = 32^°F)[/latex]
The equation, PV = nRT
where, n = number of moles in the sample of gas
R = universal gas constant;it is value 8.31 J [latex]mol^{-1} k^{-1}[/latex] known as ideal-gas equation.
It is the combination of following three laws
(i) Boyle's law :
When temperature is held constant, the pressure is inversely proportional to volume.
Boyle's law [latex]P∝[/latex][latex]\frac{1}{V}[/latex]
(ii) Charle's law :
When the pressure is held constant, the volume of the gas is directly proportional to the absolute
temperature.
Charle's law [latex]V∝T[/latex]
(iii) Avogadro's law :
When the pressure and temperature are kept constant, the volume is directly proportional to the
number of moles of the ideal gas in the container.
Avogadro's law [latex]V∝n[/latex]
Absolute Temperature
The lowest temperature of –273.16 °C at which a gas is supposed to have zero volume and zero pressure and at which entire molecular motion stops is called absolute zero temperature. A new scale of temperature starting with –273.16°C by Lord Kelvin as zero. This is called Kelvin scale or absolute scale of temperature.
T(K)=[latex]t^°C + 273.16[/latex]
The increase in the dimensions of a body due to the increase in its temperature is called thermal expansion.
Linear expansion :
The fractional increase in length per ºC rise in temperature is called coefficient of linear expansion.
Coefficient of linear expansion
[latex]α[/latex]= [latex]\frac{Δl/l}{ΔT}[/latex] = [latex]\frac{dl}{l.dT}[/latex]
Superficial expansion : On increasing the temperature of a solid, its area increases. This increase in area is referred as superficial expansion. Coefficient of superficial expansion is defined as the fractional increase in area per ºC rise in temperature.
Coefficient of a real expansion
[latex]β[/latex]= [latex]\frac{ΔA/A}{ΔT}[/latex] = [latex]\frac{dA}{A.dT}[/latex]
Cubical expansion :
On increasing the temperature of a solid, its volume increases. This increase in volume with increase in temperature is called cubical or volume expansion. Coefficient of volume expansion is defined as the fractional increase in volume per ºC rise in temperature.
Coefficient of a Volume expansion
[latex]γ[/latex] = [latex]\frac{ΔV/V}{ΔT}[/latex] = [latex]\frac{dV}{V.dT}[/latex]
Relation between coefficient of linear expansion [latex]α[/latex], coefficient
of superficial expansion [latex]β[/latex] and coefficient of cubical expansion [latex]γ[/latex]
[latex]α[/latex] = [latex]\frac{β}{2}[/latex] = [latex]\frac{γ}{3}[/latex]
[latex]α:β:γ[/latex] = 1:2:3
Anomalous Expansion of Water
Almost all liquids expand on heating but water when heated from 0°C to 4°C its volume decreases and hence density increases until its temperature reaches 4°C. Its density is maximum at 4°C on further heating its density decreases. This behaviour of water is called anomalous behaviour of water.
It is the amount of heat energy needed to raise the temperature of unit mass of substance by 1ºC (or 1K).
It is denoted by s or c.
c = [latex]\frac{1}{m}[/latex] [latex]\frac{dQ}{dT}[/latex]
Unit of specific heat capacity :
SI unit of specific heat capacity is joule/kg K
For example, the specific heat capacity of water is :
[latex]c_{water}[/latex] = 1 cal/g°C = 1 cal/g K = 1 kcal/kg K = 4200 joule/kg K
Latent Heat or Hidden Heat
When state of a substance changes, change of state takes place at constant temperature (m.pt. or b.pt.) heat is released or absorbed and is given by,
Q = mL
where L is latent heat.
The SI unit of latent heat is J/kg
Any state of a substance (solid/ liquid/ gas) can be changed into another by heating or cooling. The transition of a substance from one state to another is called a change of state.
Some common changes of states :
(i) Melting :
When heat is supplied, solid substance changes into liquid, this change of state of substance is called melting.
The temperature at which the solid and the liquid states of a substance coexist in thermal equilibrium with each other is called its melting point.
(ii) Freezing :
When heat is released, liquid changes into solid, this change of state of substance is called freezing.
(iii) Condensation :
When vapour is cooled, it changes into liquid, this change of state is called condensation
(iv) Evaporation :
Conversion of liquid into gaseous state at all the temperatures is called evaporation or boiling.
The temperature at which the liquid and vapour states of a substance coexsist in thermal equilibrium with each other is called its boiling point.
It is a phenomenon that occurs at the surface of liquids. The rate of evaporation increases with the rise in temperature. The heat required to change a unit mass of liquid into vapor at a given temperature is called heat of evaporation at that temperature.
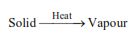
(v) Sublimation :
It is the conversion of a solid directly into vapors.
Sublimation takes place when boiling point is less than the melting point.
Heat energy transfer from a body at higher temperature to a body at lower temperature by three different methods. They are conduction, convection and radiation.
Conduction :
Conduction is that mode of transmission of heat in which heat is transferred from a region of higher temperature to a region of lower temperature by the aid of particles of the body without their actual migration. Conduction requires material medium.
Convection :
Convection like conduction requires a material medium. It is the process in which heat is transferred from one place to another by the actual movement of heated material particles.
Radiation :
When a body is heated and placed in vacuum, it loses heat even when there is no medium surrounding it. The process by which heat is lost in this case is called radiation. This does not require the presence of any material medium. It is by radiation that the heat from the sun reaches the earth.
Perfectly black body :
A black body is defined as one that will completely absorb all the radiations of whatever wavelength which falls on it. For perfectly black body, [latex]a_{\lambda}[/latex]
Properties of perfectly black body :
(i) A perfectly black body absorbs all the radiant heat incident upon it. (i.e. a = 1)
(ii) A perfectly black body does not reflect or transmit the radiant heat incident upon it. (i.e. t = 0, r = 0)
(iii) The coefficient of emission of a perfectly black body is 1. It is very good emitter of heat.
Wien's displacement law.
According to Wien’s displacement law
[latex]\lambda_m ×T[/latex]= b
Here, constant b is known as Wien's constant
The rate of cooling of a body (rate of loss of heat) is directly proportional to the excess of temp. of the body over the surroundings, provided that this excess is small and loses of heat by radiation only.
If [latex]θ and θ^0[/latex] are the temperatures of the body and its surroundings respectively, then according to Newton’s law of cooling,
Rate of loss of heat,-[latex]\frac{dQ}{dT}[/latex][latex]∝(θ-θ^0)[/latex]
The thermodynamics is the branch of science in which the conversion of heat into mechanical work and vice versa is studied.
Triple point of water :
The triple point of water represents the coexistence of all the three phases of water ice, water and water vapor in equilibrium. The pressure corresponding to the triple point of water is
[latex] 6.03×10^{-3}[/latex] atmosphere or 4.58 mm of Hg and temperature corresponding to it is 273.16 K.
Zeroth Law of Thermodynamics
If objects A and B are separately in thermal equilibrium with a third object C then objects A and B are in thermal equilibrium with each other.
Heat :
It is energy in transit between two objects or systems due to the temperature difference between them. It exists in the form of translational, rotational and vibrational motion of molecules of a substance. It depends on processes.
For constant pressure process Q =
For constant volume process Q =
For any other process Q =
where C is called molar specific heat for that process.
Internal energy :
Internal energy of a system is the energy possessed by the system due to molecular motion and molecular configuration. The energy due to molecular motion is called internal kinetic energy ([latex](U_k) [/latex] ) and that due to molecular configuration is called internal potential energy ([latex](U_p) [/latex]).
dU=[latex] dU_k [/latex] + [latex] dU_p [/latex]
If there is no intermolecular forces, then [latex] dU_p [/latex]=0
dU=[latex] dU_k [/latex] = [latex]mC_vdT [/latex]
Work :
Work is energy transfer brought about by other means, such as moving the piston of a cylinder containing the gas, by raising or lowering some weight connected to it etc.
First Law of Thermodynamics
If some quantity of heat is supplied to a system capable of doing external work, then the quantity of heat absorbed by the system is equal to the sum of the increase in the internal energy of the
system and the external work done by the system.
[latex]\delta[/latex]Q =[latex] \delta[/latex]U +[latex]\delta[/latex]W
The first law of thermodynamics is essentially a restatement of the law of conservation of energy, i.e., energy can neither be created nor be destroyed but may be converted from one form to another.
Quasi-static process :
It is infinitely slow. So its variables (P, V, T) remains in thermal and mechanical equilibrium with its surroundings.
Isochoric or isometric process :
It is a thermodynamic process that takes place at constant volume of the system, but pressure and temperature varies for change in state of the system.
Isobaric process :
It is a thermodynamic process that takes place at constant pressure, but volume and temperature varies for change in state of the system.
Isothermal process :
It is a thermodynamic process in which the pressure and volume of system change but temperature remains constant.
Adiabatic process :
It is that thermodynamic process in which pressure, volume and temperature of the system change but there is no exchange of heat between the system and the surroundings.
A process has to be sudden and quick to be adiabatic.
Equation of state :PV = [latex]\mu[/latex]RT
Equation for adiabatic process [latex]PV γ[/latex] = constant
Heat engine is a device which converts heat energy into work.
Fixed elements of a heat engine: block, cylinder head, crankcase.
Moving parts of a heat engine: piston, connecting rod, crankshaft and flywheel.
A refrigerator is the reverse of a heat engine. A heat pump is the same as a refrigerator.
The coefficient of performance of a refrigerator or heat pump.
[latex]\frac{Q_2}{W}[/latex] =[latex]\frac{Q_2}{Q_1 - Q_2}[/latex]
W = [latex] Q_1 - Q_2[/latex]
No irreversible engine (I) can have efficiency greater than Carnot reversible engine (R) working between same hot and cold reservoirs.
i.e [latex]η_R > η_l[/latex] or
1 - [latex]\frac{T_2}{T_1}[/latex] > 1 - [latex]\frac{Q_2}{Q_1}[/latex]